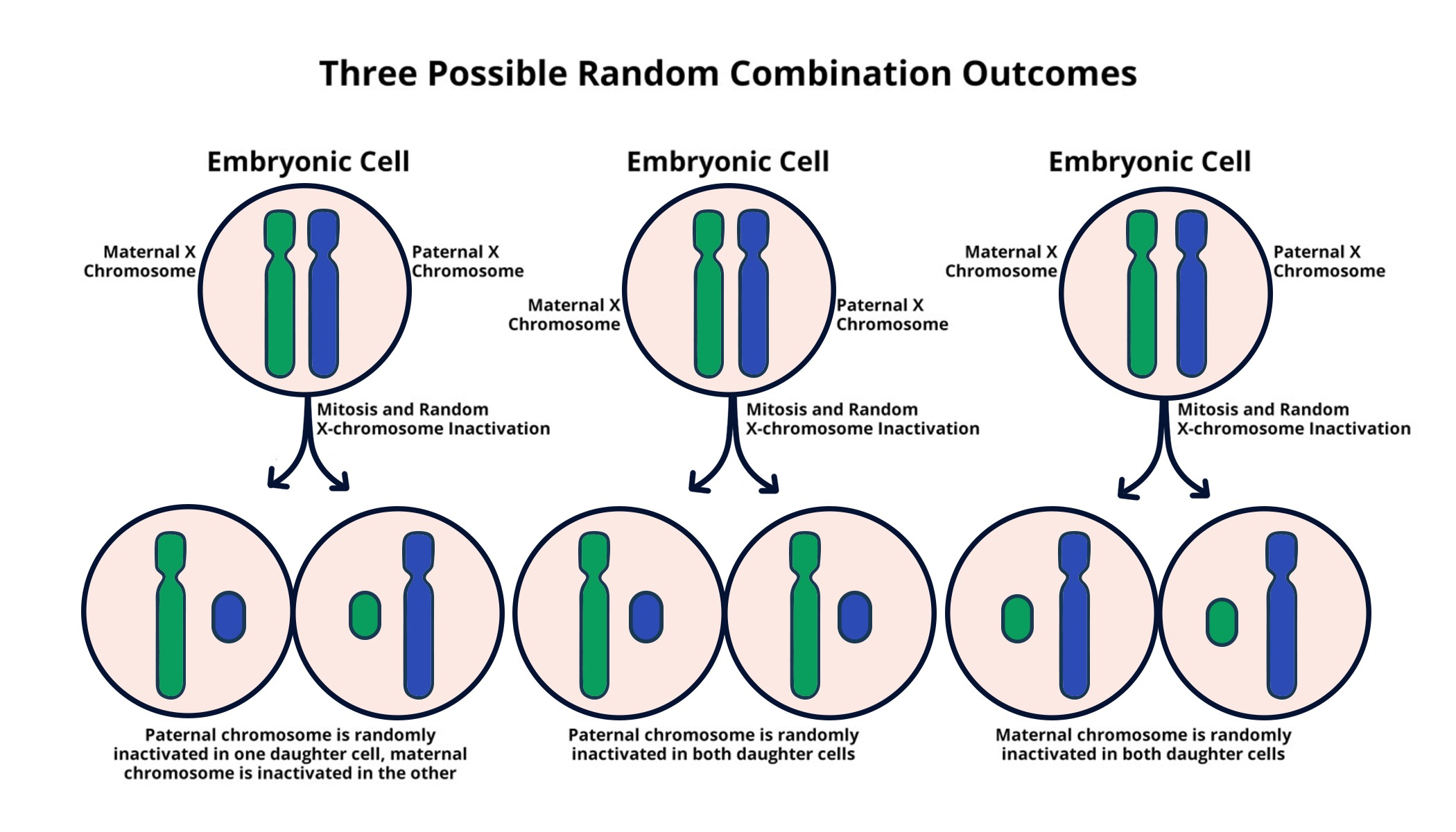
X-chromosome inactivation is a fascinating mechanism that plays a crucial role in the biology of female mammals. Unlike males, who possess only one X chromosome, females carry two, leading to a unique biological challenge: how to ensure equal gene expression from each X chromosome. This process is pivotal not only for normal cellular function but also for understanding various genetic disorders such as Fragile X Syndrome and Rett Syndrome, both linked to mutations on the X chromosome. Recent breakthroughs in chromosomal therapy have opened new avenues for treating these X-linked diseases, which affect thousands of individuals worldwide. The intricate dance of X-inactivation not only protects against gene dosage imbalances but may also hold the key to reversing effects of these debilitating conditions for future generations.
The phenomenon of X-chromosome silencing is critical in regulating gene expression in females, where one of the two X chromosomes is inactivated to prevent an overproduction of X-linked gene products. This biological process helps maintain equilibrium within the cellular environment, particularly affecting conditions associated with X-linked genetic disorders. As researchers delve deeper, they explore chromosomal regulation and potential therapeutic interventions for diseases such as Fragile X and Rett syndromes. This mechanism is a vibrant area of study, bringing hope for innovative treatments through chromosomal therapy. Understanding X-chromosome inactivation is vital, as it not only deciphering a long-standing biological puzzle but also offers potential pathways for addressing significant health challenges.
Understanding X-Chromosome Inactivation and Its Implications
X-chromosome inactivation (XCI) is a crucial process that ensures dosage compensation for genes located on the X chromosome in females. Since females have two X chromosomes while males only possess one, one of the X chromosomes in females becomes largely inactive. This genetic phenomenon is vital for balancing the expression of X-linked genes, thereby preventing overexpression in females, which could lead to disorders. Research led by Jeannie Lee at Harvard Medical School has illuminated the molecular mechanisms involved in this process, highlighting the role of a gelatinous substance that coats chromosomes, effectively segregating them and facilitating the inactivation necessary for genetic stability.
The significance of X-chromosome inactivation extends beyond mere genetic regulation; it opens avenues for therapeutic interventions in X-linked diseases. Notably, conditions such as Fragile X Syndrome and Rett Syndrome arise from mutations on the X chromosome. By understanding how XCI occurs, researchers have begun exploring ways to ‘unsilence’ inactivated X chromosomes, providing potential relief for affected individuals. This research fosters hope for innovative treatments that could harness the natural mechanisms of gene regulation to combat these debilitating genetic disorders.
The Role of Chromosomal Therapy in Treating Genetic Disorders
Chromosomal therapy represents an advanced therapeutic strategy aimed at correcting genetic disorders by targeting the molecular structures of chromosomes themselves. This innovative approach leverages the principles of gene therapy and chromosomal biology to restore the function of mutated genes. As highlighted in the findings by Jeannie Lee, manipulating the state of X-chromosome inactivation could provide a dual benefit: silencing detrimental mutations while preserving the functionality of healthy genes. Furthermore, this technique could represent a paradigm shift in how we address genetic conditions, especially those that predominantly affect female patients due to X-linked inheritance patterns.
Recent advancements in chromosomal therapy underscore its potential to unlock new treatment pathways for disorders linked to mutations that reside on the X chromosome. For instance, by employing methods to release inactivated genes, researchers can potentially reactivate healthy copies of genes impaired by mutations, as seen in Fragile X and Rett syndromes. The promise of this therapeutic landscape lies not only in improving patient outcomes but also in minimizing the side effects often associated with broader genetic interventions, making this a field to watch as future innovations unfold.
Exploring the Link Between X-Chromosome Inactivation and X-Linked Diseases
Understanding the intricate relationship between X-chromosome inactivation and X-linked diseases is fundamental to advancing treatments for conditions like Fragile X Syndrome and Rett Syndrome. These diseases are directly attributed to mutations on the X chromosome, and the mechanism of XCI offers a unique perspective on how these mutations can be managed. Since females carry two X chromosomes, abnormalities in one can, under normal circumstances, be masked by a functioning copy on the other chromosome. However, if both copies are affected or one is inactivated inappropriately, it can lead to the manifestation of these disorders, highlighting the critical nature of XCI in healthy gene function.
Dr. Jeannie Lee’s research into the mechanisms of chromosomal behavior has the potential to revolutionize how we approach the treatment of X-linked diseases. By targeting the process of X-inactivation, scientists could devise strategies to restore activity to silenced genes that hold the key to curing these genetic disorders. The ongoing studies of chromosomal therapy not only emphasize the need for detailed understanding but also inspire hope for viable treatments that could diminish the impact of these challenging diseases on affected individuals and families.
Innovative Approaches to Gene Therapy for Fragile X and Rett Syndromes
Gene therapy has emerged as a promising approach for treating genetic disorders, particularly X-linked diseases such as Fragile X Syndrome and Rett Syndrome. By directly targeting the genetic mutations responsible for these conditions, researchers aim to restore normal gene function and alleviate symptoms. Recent discoveries regarding X-chromosome inactivation have invigorated the quest for effective gene therapies, providing insights into how we might reactivate healthy genes that have been silenced in individuals with these diseases. The versatility of gene therapy combined with the knowledge of chromosomal behavior opens a pathway to novel treatment options.
Current research efforts are focused on developing effective delivery methods for gene therapies, ensuring that they reach the intended cells without causing significant side effects. This is particularly crucial for conditions like Fragile X Syndrome, where the mutation may be present on one X chromosome while the other remains functional. By harnessing the mechanisms of X-chromosome inactivation, innovative therapies could selectively target the affected genes, thereby minimizing risks while maximizing therapeutic success. As the landscape of genetic medicine continues to evolve, the integration of chromosomal therapy could play a vital role in reshaping treatments for these debilitating disorders.
The Future of Chromosomal Research and Its Therapeutic Potential
The future of chromosomal research is poised for significant breakthroughs, particularly with the emergence of therapies targeting genetic disorders like Fragile X and Rett syndromes. As researchers delve deeper into the mechanics of X-chromosome inactivation, the potential to develop effective therapies increases exponentially. These discoveries not only help elucidate the fundamental biology behind X-linked diseases but also propose avenues for innovative treatments that could ultimately change the lives of affected individuals. The focus on chromosomal dynamics—how chromosomes interact with their environment—provides a fresh perspective on traditional genetic therapies.
Continued funding and support for research initiatives are essential for realizing the full potential of these discoveries. With pioneers like Jeannie Lee at the helm, the exploration of chromosomal therapy could unlock new strategies for managing genetic disorders. The ability to manipulate gene expression through controlled release or inactivation of excess genes could lead to personalized medicine solutions that cater to the unique genetic makeup of each patient. As we edge closer to realizing these advancements, the therapeutic implications for individuals suffering from X-linked diseases remain a thrilling frontier in genetics.
Chromosomal Breakthroughs: Insights from Research
Recent advancements in chromosomal research have provided insights that hold great promise for treating genetic disorders, particularly X-linked conditions. Groundbreaking studies, such as those undertaken by Dr. Jeannie Lee, have unveiled the complexities of X-chromosome inactivation, a critical process that ensures genetic balance within cells. By understanding how chromosomes interact and how silencing mechanisms function, scientists are beginning to formulate strategies that could effectively target and treat disorders like Fragile X and Rett syndromes. This knowledge can lead to the development of therapies that not only address symptoms but also aim to cure the underlying genetic causes.
The excitement surrounding these chromosomal breakthroughs stems from their potential applications in clinical settings. As researchers continue to decipher the cellular machinery behind X chromosome behavior, therapy development becomes increasingly feasible. Future studies may explore how these newly discovered mechanisms can be translated into practical treatments, possibly allowing women with X-linked disorders to tap into the genetic resources trapped within their silenced chromosomes. This innovative marriage of chromosomal biology and therapeutic development could herald a new era in the treatment of genetic disorders.
Challenges and Opportunities in Targeting X-Linked Diseases
Targeting X-linked diseases such as Fragile X Syndrome and Rett Syndrome presents both challenges and opportunities for researchers and clinicians alike. Diagnosing these conditions can be complicated, often requiring comprehensive genetic testing to identify mutations. Additionally, the mutations associated with these disorders can manifest differently across individuals, making treatment strategies complex. However, the determination of mechanisms like X-chromosome inactivation offers a pathway for targeted therapies that could address the root causes of these conditions and potentially restore functionality to affected genes.
Equipped with insights from chromosomal research, researchers are optimistic about overcoming these challenges. For instance, targeted chromosomal therapies that focus specifically on restoring the function of silenced genes have the potential to provide significant relief for patients. The ability to harness natural biological processes, like those governing XCI, creates room for innovative approaches in addressing X-linked diseases. As the field progresses, continued collaboration between geneticists, biologists, and clinicians will be crucial to translating these findings into practical treatments that can improve the quality of life for people affected by genetic disorders.
Understanding Fragile X Syndrome and Its Genetic Basis
Fragile X Syndrome (FXS) is one of the most common inherited forms of intellectual disability and is caused by a mutation on the FMR1 gene located on the X chromosome. This mutation leads to the absence of a crucial protein, FMRP, that is important for normal neural development. Children with FXS often display behavioral challenges, learning difficulties, and developmental delays. Understanding the genetic basis of FXS, including how it interacts with X-chromosome inactivation mechanisms, is pivotal in advancing our approaches to prevention and treatment.
Recent studies indicate that the presence of two X chromosomes in females can sometimes mitigate the severity of symptoms associated with FXS. However, if both chromosomes harbor the mutation or if one is inactivated, the implications for cognitive and emotional development can be profound. This highlights the importance of research focused on XCI, as unlocking the potential for reactivation of silenced genes may offer new therapeutic strategies to alleviate the effects of FXS. As science advances, there is hope for targeted therapies that could enhance the lives of those living with this condition.
Rett Syndrome: Genetic Underpinnings and Therapeutic Prospects
Rett Syndrome is a neurodevelopmental disorder primarily affecting girls, characterized by a period of normal development followed by a loss of skills and severe cognitive impairment. It is usually caused by mutations in the MECP2 gene located on the X chromosome. Like Fragile X Syndrome, understanding the genetic mechanisms underlying Rett Syndrome requires a comprehensive look at X-chromosome inactivation and its implications for gene expression. The loss of MECP2 function disrupts critical neural circuits, leading to the hallmark symptoms of the disorder.
Researchers are increasingly optimistic that studying the process of X-inactivation can yield therapeutic opportunities for treating Rett Syndrome. By exploring methods to target the inactivated X chromosome, researchers may restore the necessary gene function and palliate the symptoms associated with the disorder. Innovative therapies that focus on demethylation of the MECP2 gene or unsilencing its expression could represent a future path towards significant improvements in quality of life for those affected by Rett Syndrome. As this research evolves, it underscores the importance of genetic insight in shaping treatment options.
Frequently Asked Questions
What is X-chromosome inactivation and why is it important in understanding X-linked diseases?
X-chromosome inactivation (XCI) is a biological process where one of the two X chromosomes in females is randomly inactivated, thus ensuring that females, like males, have only one functional copy of the X chromosome. This process is crucial for understanding X-linked diseases, such as Fragile X Syndrome and Rett Syndrome, as it impacts gene expression and potential treatments based on the availability of healthy gene sequences.
How does X-chromosome inactivation relate to Fragile X Syndrome?
Fragile X Syndrome is caused by mutations on the X chromosome. During X-chromosome inactivation, one of the X chromosomes is silenced, often rendering the healthy gene inactive. Understanding how XCI works allows researchers to explore therapeutic approaches that could potentially reactivate these silenced genes and alleviate symptoms associated with Fragile X Syndrome.
Can chromosomal therapy target X-chromosome inactivation to treat genetic disorders like Rett Syndrome?
Yes, chromosomal therapy has the potential to target X-chromosome inactivation (XCI) mechanisms to treat genetic disorders such as Rett Syndrome. By developing methods to unsilence the inactive X chromosome, researchers aim to access the healthy genes that can compensate for the mutated genes causing these X-linked diseases.
What are the implications of X-chromosome inactivation for males with X-linked diseases?
While males have only one X chromosome and do not undergo X-chromosome inactivation, they can still be affected by mutations on that chromosome, such as those leading to Fragile X Syndrome. Research on XCI has revealed that the same mechanisms can also silence specific genes on the X chromosome in males, offering potential avenues for therapeutic intervention.
How does the discovery of ‘Jell-O-like’ substances enhance our understanding of X-chromosome inactivation?
The discovery of a gelatinous substance that coats chromosomes provides insight into how X-chromosome inactivation occurs. This ‘Jell-O-like’ material facilitates the silencing of one X chromosome by changing its properties through the action of molecules like Xist. Understanding these interactions opens new pathways for treating diseases caused by mutations on the X chromosome, offering hope for conditions like Fragile X Syndrome and Rett Syndrome.
What role does the RNA molecule Xist play in X-chromosome inactivation?
Xist, an RNA molecule produced from the X chromosome, initiates X-chromosome inactivation by coating the chromosome and modifying its surrounding ‘Jell-O-like’ material. This process changes the biophysical properties of the inactivated X chromosome, rendering its genes inactive. Insights into Xist’s function are critical for developing therapies to reactivate genes in genetic disorders related to X-linked mutations.
What progress has been made in making X-chromosome inactivation reversible for therapeutic use?
Recent studies have identified methods to reverse X-chromosome inactivation, allowing for the restoration of function to mutated genes associated with conditions like Fragile X Syndrome and Rett Syndrome. Researchers in Jeannie T. Lee’s lab are working on optimizing these approaches, with plans for future clinical trials to explore their safety and efficacy in treating X-linked diseases.
| Key Point | Details |
|---|---|
| X-Chromosome Inactivation | Females have two X chromosomes, and one is inactivated to balance gene dosage with males who have one X chromosome. |
| Role of Xist RNA | Xist RNA is crucial for initiating the inactivation process by altering the properties of the surrounding chromosomal structure. |
| Jell-O-like Substance | A gelatinous substance helps maintain separation between chromosomes and facilitates the interaction necessary for inactivation. |
| Therapeutic Potential | Understanding the inactivation process may lead to treatments for diseases like Fragile X and Rett syndrome, particularly by unsilencing genes. |
| Clinical Research | Jeannie Lee’s lab is working towards clinical trials for therapies based on their findings on X chromosome inactivation. |
Summary
X-chromosome inactivation is a fundamental biological process whereby females inactivate one of their two X chromosomes to achieve gene dosage balance with males. This crucial mechanism, discovered by researchers like Jeannie T. Lee, involves complex interactions between molecules like Xist RNA and a unique gelatinous substance that preserves chromosomal structure. The insights gained from understanding X-chromosome inactivation not only deepen our knowledge of genetic regulation but also hold the promise for developing innovative treatments for genetic disorders such as Fragile X and Rett syndromes. By potentially freeing inactivated X chromosomes, therapeutic approaches may provide crucial benefits to individuals affected by these conditions.